Allosteric Regulation Is Most Similar to Which of the Following
Diffrent allosteric regulators turn enzyme activity on or off by binding the same site. As far as the transduction of heterotropic effects is concerned the following pathways have been proposed for ATP signaling.
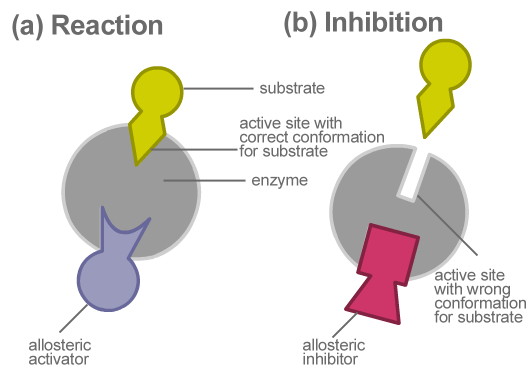
Allosteric Inhibition The Definitive Guide Biology Dictionary
Allosteric regulation of which of the following enzymes is important in the regulation of glycolysis.
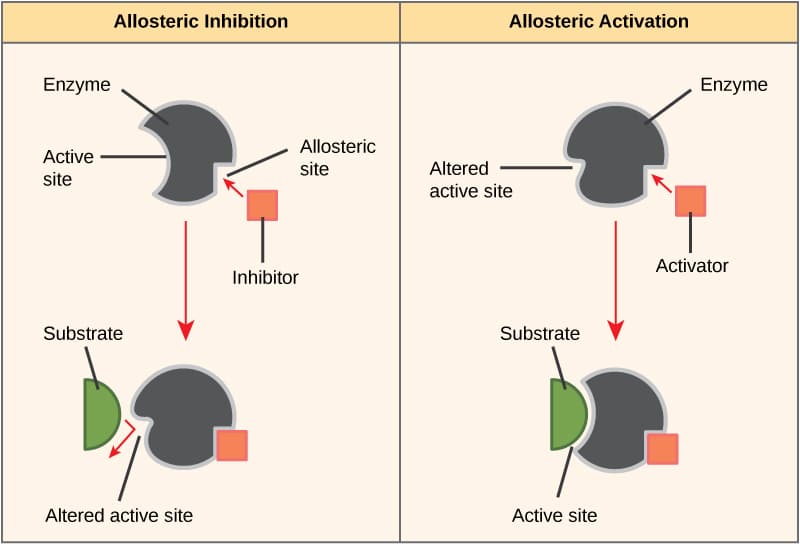
. In most cases the binding. The enzyme is subject to competitive inhibition and allosteric regulation. Regulatory molecules bind to a site remote from the active site.
It is usually the mode of regulation for the last step in reaction pathways since this step produces the final product. Which statement is false about allosteric regulation. In biochemistry allosteric regulation is the regulation of an enzyme or protein by binding an effector molecule at the proteins allosteric site that is a site other than the proteins active site.
Allosteric enzymes are rarely important in the regulation of metabolic pathways. They can modulate their effect in a more subtle way b. In biochemistry allosteric regulation is the regulation of an enzyme by binding an effector molecule at a site other than the enzymes active site.
During the heyday of metabolic research allosteric regulators were actively sought out. They may be safer as they have no effect at all unless the natural substrate is present d. The answer is B.
Which of the following is most likely to be correct. Effectors that enhance the proteins activity are referred to as allosteric activators whereas those that decrease the proteins activity are called allosteric inhibitors. The sgRNA functional modules are defined as in ref.
It expands this site and thereby influences neighboring residues. Allosteric regulations is always used to negatively regulate enzyme activity. Allosteric regulation is the balance of enzyme activity using specialized molecules that affect the enzymes.
Explore the process of the allosteric regulation of enzymes and how feedback inhibition. A mimic of the substrate competes for the active site. Which of the following statements best describes allosteric regulation.
Binding of allosteric regulators alters the conformation of an enzyme. In that it is defined and named from a negative point of view. Which of the following is true.
C A trace metal binds to an enzyme and a substrate likning the 2 together. Allosteric regulations are often end products of a biochemical pathway. A Domain organization of Cas9b Schematic representation of the Cas9 sgRNA base pairing with target DNA.
Cas9 is subjected to allosteric regulation at different assembly stages following. When an effector molecule binds to an allosteric site the enzyme changes conformation causing the enzyme to have a high affinity for its ligand at the active site. Allosteric regulation of enzymes is crucial for the control of cellular metabolism.
Allosteric sites allow effectors to bind to the protein often resulting in a conformational change involving protein dynamics. In competitive the substrate and inhibitor bind at the same active site - pretty straightforward. B Two types of allosteric regulation occur.
In most allosteric proteins. Which of the following statements about allosteric enzyme regulation are true. Allosteric inhibition is designed into the proteins and represents an important physiological process.
Two of the earliest examples of allosteric regulation are the control of oxygen binding to hemoglobin by protons 1 and AMP activation of glycogen phosphorylase 2. The subunits of the hemoglobin have allosteric effects on each other. ATP binds to the allosteric site in a slightly different way from CTP.
Allosteric enzyme regulation therefore is when a molecule binds a site other than the active site and changes the behavior of the enzyme by changing its conformation. In allosteric regulation speaking specifically about inhibition here the inhibitor is binding at a site other than the active site and changing the enzyme in some way to make it inactive. All of these are advantages e.
Allosteric regulators are often end products of a biochemical pathway. Noncompetitive inhibition is more of a catch-all for non-physiological inhibition that does not compete with substrate for substrate binding to enzyme. Which statement is false about allosteric regulation.
C Diverse allosteric modulations leading to CRISPR-Cas9 with altered properties. The binding of one molecule activates the enzyme while the binding of a different molecule inhibits it. A good example to explain allosteric regulation is hemoglobin which is not an enzyme though.
B Coenzymes bind to a protein changing its activity. The enzyme contains α-helices and β-pleated sheets. Allosteric communication between distant sites in proteins is central to biological regulation but still poorly characterized limiting understanding engineering and drug development 123456.
Allosteric regulation is always used to negatively regulate enzyme activity. An allosteric drug may be more specific for specific reaction than one that binds to the receptor itself c. Allosteric Regulation of Catalytic Activity.
Allosteric enzymes have a hyperbolic plot of reaction rate vs. A Rate of protein synthesis is changed by binding or a regular molecule to the promoter sequence. The site to which the effector binds is termed the allosteric site or regulatory site.
A The enzyme contains α-helices and β-pleated sheets. Yes but it helps to be more specific. Michaelis-Menten kinetics describe the reactions of allosteric enzymes C.
A naturally occurring molecule stabilizes a catalytically active conformation. Allosteric regulation of CRISPR-Cas9. Which of the following characteristics is not associated with allosteric regulation of an enzymes activity.
Different allosteric regulators turn enzyme activity on or off by binding the same site. Allosteric regulation occurs when an activator or inhibitor molecule binds at a specific regulatory site on the enzyme and induces conformational or electrostatic changes that either enhance or reduce enzyme activity. Hexokinase PFK-1 Pyruvate kinase Both A and C are correct All of the above are correct Which of the following molecules can be used to synthesize glucose via Lactate Pyruvate Glycerol Keto acids All of the above are correct The enzymes involved in shutting.
Cellular response is faster with allosteric controlthan by controlling enzyme concentration in the cell. Either the enzyme has two distinct active sites or the substances involved in the two reactions have very similar structures. Which of the following is an advantage of an allosteric drug compared to an orthosteric one.
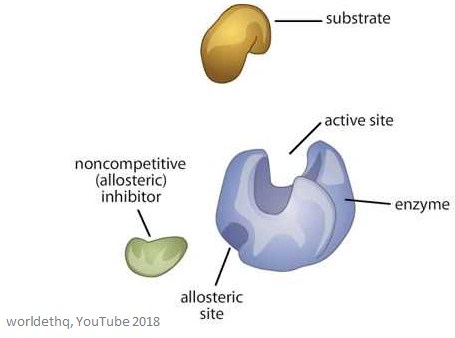
Allosteric Enzymes Facts Summary Definition Chemistry Revision

Enzyme Inhibition Types Of Inhibition Allosteric Regulation Teachmephysiology
0 Response to "Allosteric Regulation Is Most Similar to Which of the Following"
Post a Comment